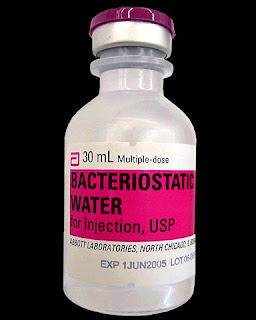
Bacteriostatic Water for mixing in hCG injections
Bacteriostatic Water
Bacteriostatic Water Information
Bacteriostatic Water is sterile water containing 0.9% benzyl alcohol that is used to dilute or dissolve medications; the container can be reentered multiple times and the benzyl alcohol suppresses or stops the growth of most potentially contaminating bacteria. This
preparation of sterile water and benzyl alcohol (BnOH) allows repeated
withdrawals to be made from a single 30mL plastic vial. Hospira, the world’s
leading manufacturer and supplier of bacteriostatic and sterile water,
recommends that once a vial of bacteriostatic water is open the preservatives
will prolong its life for up to 28 days and that after this period of time the
vial should to be discarded. Other sterile waters for injection without the
bacteriostatic agent (single-dose vials) should be discarded after a single
use. Visit our buy Bacteriostatic Water page to purchase online without a prescription.
Bacteriostatic Water Warnings
Benzyl alcohol, a preservative in
Bacteriostatic Water for Injection, USP has been associated with toxicity in
neonates. Data are unavailable on the toxicity of other preservatives in this
age group. Where water is required for preparing or diluting medications for
use in neonates, only preservative-free Sterile Water for Injection should be
used.
Intravenous administration of
Bacteriostatic Water for Injection without a solute may result in hemolysis.
PRECAUTIONS
Do not use Bacteriostatic Water for Injection, USP for
intravenous injection unless the osmolar concentration of additives results in
an approximate isotonic admixture.
Consult the manufacturer’s instructions for choice of vehicle,
appropriate dilution or volume for dissolving the drugs to be injected,
including the route and rate of injection.
Inspect reconstituted (diluted or dissolved) drugs for clarity
(if soluble) and freedom from unexpected precipitation or discoloration prior
to administration.
Pregnancy Category C. Animal
reproduction studies have not been conducted with Bacteriostatic Water for
Injection. It is also not known whether Bacteriostatic Water for Injection
containing additives can cause fetal harm when administered to a pregnant woman
or can affect reproduction capacity. Bacteriostatic Water for Injection, USP
containing additives should be given to a pregnant woman only if clearly
needed.
Pediatric Use
The safety and effectiveness of Bacteriostatic Water for
Injection, USP have not been established in pediatric patients. Due to the
potential for toxicity, solutions containing benzyl alcohol should not be used
in neonates.
Bacteriostatic Water Interactions
Some drugs for injection may be incompatible in a given vehicle,
or when combined in the same vehicle or in a vehicle containing benzyl alcohol.
Consult with pharmacist, if available.
Use aseptic technique for single or multiple entry and
withdrawal from all containers.
When diluting or dissolving drugs, mix thoroughly and use
promptly.
Do not store reconstituted solutions of drugs for injection
unless otherwise directed by the manufacturer of the solute.
Do not use unless the solution is clear and seal intact.
Bacteriostatic Water Overdose
Use only as a diluent or solvent.
This parenteral preparation is unlikely to pose a threat of fluid overload
except possibly in very small infants. In the event these should occur,
re-evaluate the patient and institute appropriate corrective measures.
Buy Bacteriostatic Water
Bacteriostatic Water for mixing in hCG injections
 Reviewed by Sifasi Injection
on
16:30
Rating:
Reviewed by Sifasi Injection
on
16:30
Rating:
 Reviewed by Sifasi Injection
on
16:30
Rating:
Reviewed by Sifasi Injection
on
16:30
Rating:












No comments: